Wang Lab

Investigators in the Wang Lab at NYU Langone aim to understand the mechanisms underlying Na+-driven tri- and dicarboxylate transporters in the plasma membrane. In human cells, cytosolic citrate is a chief precursor for the synthesis of fatty acids, triacylglycerols, cholesterol, and low-density lipoprotein. Cytosolic citrate further regulates the energy balance of the cell by activating the fatty-acid-synthesis pathway while downregulating both the glycolysis and fatty-acid β-oxidation pathways.
The rate of fatty-acid synthesis correlates directly with the concentration of citrate in the cytosol, with the cytosolic citrate concentration partially depending on direct import across the plasma membrane through the Na(+)-dependent citrate transporter (NaCT). Mutations of the homologous fly gene INDY (I'm Not Dead Yet) result in reduced fat storage through calorie restriction. NaCT-knockout mice have increased hepatic mitochondrial biogenesis, higher lipid oxidation and energy expenditure, and reduced lipogenesis, protecting the mice from obesity and insulin resistance.
We have determined the crystal structure of a bacterial INDY homologue. One citrate molecule and one sodium ion are bound per protein, and their binding sites are defined by conserved amino acid motifs, forming the structural basis for understanding the specificity of the transporter. Comparison of the structures of the two symmetrical halves of the transporter suggests conformational changes that propel substrate translocation.
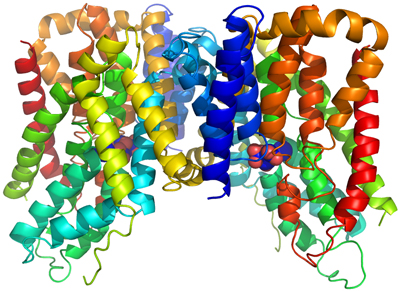
In 2009, we determined the structures of the formate channel FocA from Vibrio cholerae, without and with formate ions bound. The ion selectivity filter in FocA consists of a cytoplasmic slit and a central constriction ring. Interactions of the filter with bound formate ions provide a structural basis for the ion selectivity of the channel. The structures also suggest a possible gating mechanism in which movements of a cytosolic loop open and close the channel. This is the first time that the ion selectivity and gating mechanism was understood for an organic-ion channel.

In 2003, we solved the structure of the glycerol-3-phosphate transporter (GlpT) from the E. coli inner membrane. The protein belongs to the major facilitator superfamily (MFS), the largest secondary transporter family with more than 100,000 identified members, including 107 genes in the human genome. Together with the lactose permease structure, this is the first MSF structure ever determined to high resolution. The transporter structure suggests that the substrate translocation is by alternating-access via a rocker-switch mechanism.
Contact Us
Da-Neng Wang, PhD
Professor, Department of Cell Biology
540 First Avenue
Third Floor, Lab 5
New York, NY 10016
Phone: 212-263-8634